

NEWS
2025.12.15
Completion of Patent Examination and Patent Grant Procedures for Drug Delivery System “Perfusio”
Veritas In Silico, Inc. (hereinafter referred to as “VIS”) is advancing efforts to create new mRNA-targeted pharmaceuticals (pipeline creation) in-house. As part of an initiative to address rare diseases with Unmet Medical Needs using nucleic acid therapeutics as the appropriate modality and leveraging the strength of our technology being applicable to both small molecule drug discovery and nucleic acid therapeutics discovery, we are advancing the research and development of nucleic acid therapeutics as our first pipeline candidate.
VIS had conceived and filed a patent application for a Drug Delivery System*1 (hereinafter referred to as “DDS”; product name: “Perfusio”) necessary for administering nucleic acid therapeutics. It is hereby announced that the examination by the Patent Office has been completed and the patent grant procedure has been finalized.
・Application Number: JP 2025-086160
・Title of Invention: Method for Local Administration of Medication
・Patent Applicant: Veritas In Silico Inc.
・Patent Grant Date: October 21, 2025
Nucleic acid therapeutics are typically costly, making the precise delivery of the drug to the target disease organ a critical aspect of the treatment. Therefore, there is a demand for DDS that can be applied to all nucleic acid therapeutics. This technology should enable precise administration to various target organs and prevent the drug from reaching organs other than the intended target.
The DDS patent that has been obtained this time utilizes a dual-structure balloon catheter that was developed by our company. This catheter is designed with a unique structure that utilizes a balloon to seal the blood vessel, with a catheter capable of flow positioned both before and after the occlusion. (Figure 1)
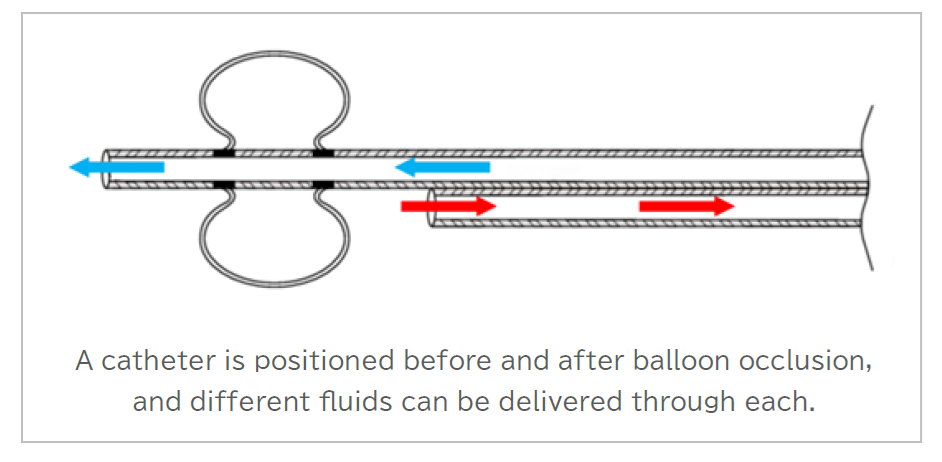 (Figure 1) Example of a double-balloon catheter cross-sectional structure
(Figure 1) Example of a double-balloon catheter cross-sectional structure
A key benefit of this approach is that it allows for Organ Perfusion*2 using a non-blood fluid while the target organ remains within the human body. This is achieved by placing the catheter separately on the venous and arterial sides of the target organ and occluding them. If the perfusion fluid contains medication, that pharmaceutical is delivered exclusively to the target organ and subsequently retrieved through the catheter. (Figure 2)
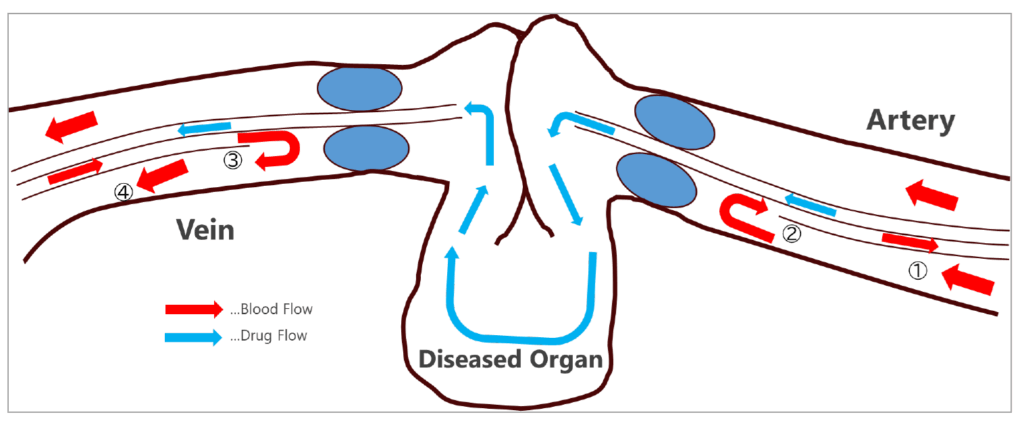 (Figure 2) Schematic diagram of organ perfusion using a double-balloon catheter
(Figure 2) Schematic diagram of organ perfusion using a double-balloon catheter
The use of this catheter enables selective and precise drug delivery to the target organ. This approach also facilitates the administration of drugs to the minimum required amount, ensuring a streamlined and efficient process. We are pursuing early practical application by combining it with the simple nucleic acid therapeutics we are developing in our own pipeline. This approach has the potential to offer new treatment options for various organs and diseases while maintaining cost-effectiveness.
■Comments from Shingo NAKAMURA, PhD, Representative Director and CEO of VIS
Our DDS is an ambitious and innovative delivery technology for nucleic acid therapeutics. It facilitates highly precise delivery, including to organs previously considered inaccessible, such as the left or right kidney, the left or right lung, and ultimately, even a specific part of the lung. This approach also holds promise for eliminating liver toxicity, the primary concern during administration of nucleic acid therapeutics.
Additionally, the available drugs encompass more than just nucleic acid therapeutics. The delivery of small molecule drugs and antibody drugs is also a possibility. From this perspective, this DDS can be readily applied to treatments using already approved drugs as soon as regulatory approval is obtained.
The utilization of this DDS has the potential to significantly reduce the research and development period for our proprietary nucleic acid therapeutic pipeline. This initiative aims to expedite the delivery of novel treatment options to patients.
Building on this technology, we will intensify our focus on proprietary drug discovery research, aiming to contribute to the realization of a “warm society filled with hope.”
■Impact on Future Business Performance of VIS
This DDS will be commercialized going forward by the ” Corporate Venture Office ” to be established within VIS on January 1, 2026, under a plan that includes intellectual property strategy. VIS is currently examining the potential for this to have a significant impact on our performance for the fiscal year ending December 2026 and beyond.
In case any matters requiring disclosure arise in the future, they will be promptly disclosed.
■Glossary for Reference
*1 Drug Delivery System: This system has been developed to administer the active pharmaceutical ingredients to the intended target organs. Typically, methods such as chemically attaching molecules that selectively reach the target organ to the drug or encapsulating the drug within a lipid bilayer membrane are used. However, our company considers physically delivering the drug—by approaching the target organ via an arterial catheter and additionally approaching it via a venous catheter—to be one form of drug delivery system.
*2 Organ Perfusion: The deliberate infusion of blood, medication, or fluids containing oxygen and nutrients into a specific organ.

